Manufacturing

Mylan has a vast manufacturing platform in India which comprises active pharmaceutical ingredient (API), oral solid dose (OSD) and injectables facilities that serve a number of markets around the world and produce medicines in a wide range of therapeutic areas.
Seventeen of Mylan’s facilities in India, including all of its API facilities, are approved by the U.S. Food and Drug Administration (FDA). Many of these sites also are approved and regularly inspected by other regulatory authorities, including Australia's Therapeutic Goods Administration, the U.K.’s Medicines and Healthcare Products Regulatory Agency and the World Health Organization.
R&D Network
Mylan’s globally integrated R&D platform helps expand our product portfolio to satisfy unmet medical needs. We have < 3000 scientific affairs workforce who work collaboratively across multiple R&D centers around the world. About half of our scientific affairs workforce is based in India.
Active Pharmaceutical Ingredients
Mylan is one of the world's largest API manufacturers as measured by the number of drug master files on record with regulatory agencies. Mylan uses the APIs it develops to supply its internal network and to sell to third parties. The company operates all nine of its API facilities in India and offers a broad portfolio and pipeline of more than 250 APIs in a variety of therapeutic categories, from antibacterials to antifungals. Mylan has an API capacity of >4800 kiloliters.
REDUCING EFFLUENT DISCHARGE – LEADING BY EXAMPLE
Responsible wastewater treatment is a key topic for our industry and Mylan is committed to leading by example. The production requirements of our operations – coupled with local regulations and infrastructure – guide the type of water and wastewater management applied. In India, we are a leader in zero liquid discharge (ZLD) systems. ZLD systems are wastewater treatment plants that eliminate effluent discharge into the environment. Instead, all wastewater is treated and repurposed back into non-potable applications in our facilities, reducing water usage and significantly reducing environmental impact.
Oral Solid Doses
Mylan operates seven global state-of-the-art finished dosage form facilities in India that manufacture OSD, like tablets and capsules, across a wide range of therapeutic categories, such as antibacterials, antidiabetics, antiretrovirals, cardiovascular therapies and central nervous system agents. These sites are located in Aurangabad, Indore, Jadcherla, Sarigam, Ahmedabad, Jaggiapet, and Nashik.
Mylan India manufactures and supplies generic and branded generic OSD products from India to various markets, including Australia, Canada, Europe, India, Japan, New Zealand and the U.S. The sites are routinely inspected by health authorities around the world noted in the below table, including the U.S. Food and Drug Administration, European regulatory authorities, the World Health Organization and many others. We also manufacture our OSD antiretroviral products for markets in India and emerging markets.
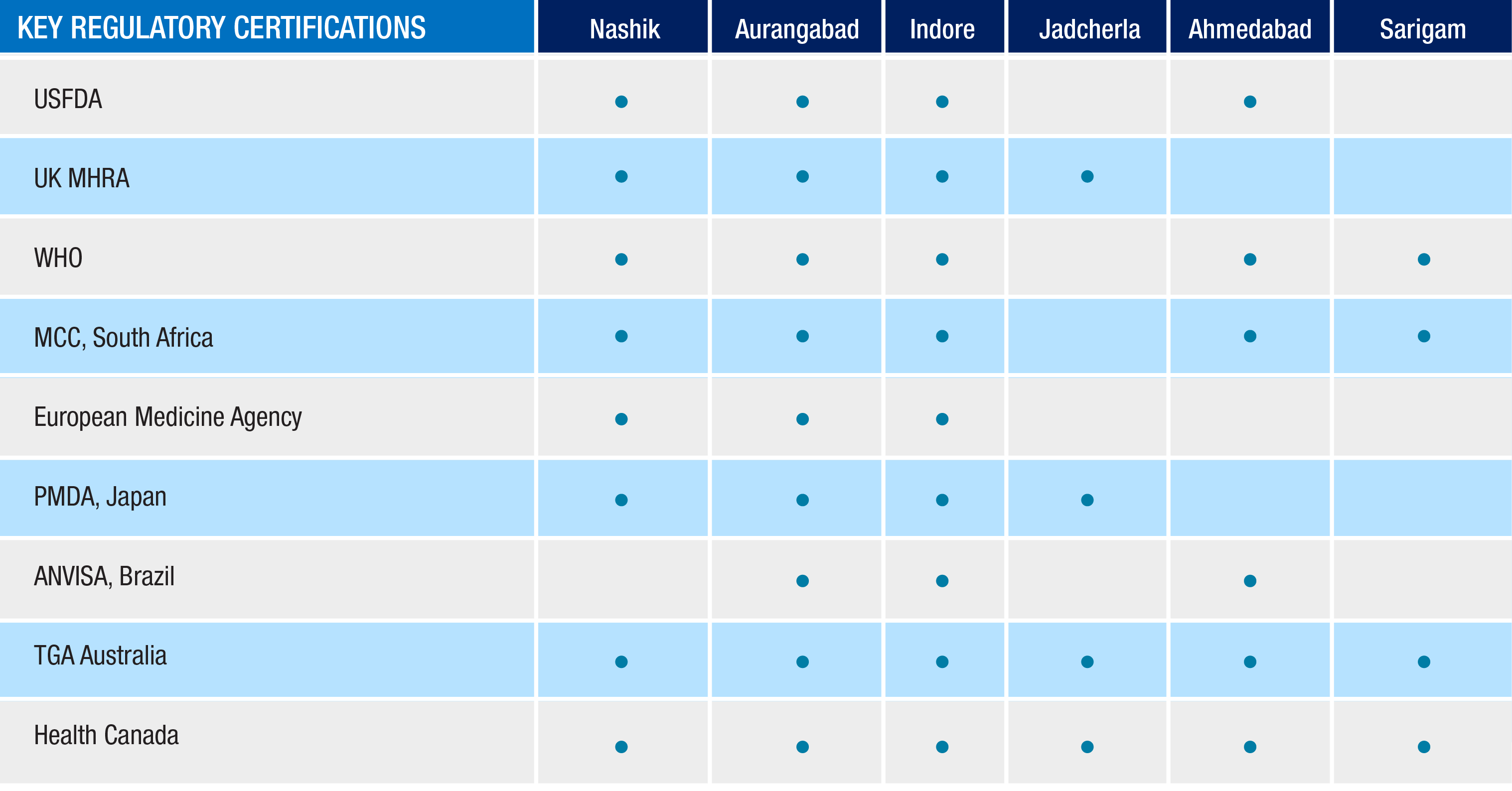
Key regulatory approvals :
Dosage forms
- Capsules filled with powder and pellets
- Immediate-release tablets
- Modified-release tablets
- Sachet filled with powder
Packaging capabilities
- Blister packaging
- Blister pouch
- Blister co-pack
- Bottle packaging
Manufacturing capabilities
- Bi-layered tablets
- Capsules banding
- Direct compression
- Dry granulation
- Extrusion spheronization
- Fluid bed drier
- Hot melt extrusion
- Laser drilling
- Multiple-unit pellet system
- Pellets and beads
- Roller compaction
- Solid drug layering
- Tablet coater
- Wet granulation
- Wurster coater with capability to handle solvents
Injectables
In 2013, Mylan became a global injectables leader with the acquisition of Agila Specialties. The transaction significantly expanded the company’s injectable manufacturing capabilities in India. Today, Mylan operates seven injectables facilities, five of which are based in India. The company offers a robust portfolio of high-quality specialty and generic injectables in a variety of important therapeutic categories, such as oncology, anti-infective and cardiovascular. Mylan sells these products around the world including Brazil, Canada, Europe, Japan and the U.S.
Our technological capabilities have been enhanced significantly with our industry-leading sterile manufacturing, state-of-the-art lyophilization process and diverse delivery systems, which include:
- Lyophilized and liquid vials
- pre-filled syringes
- ampoules and
- mini bags.