Mylan is first to offer innovative, reduced-dose HIV treatment in India
WHO recommended antiretroviral is helping to expand access to patients
in India at a lower cost
HERTFORDSHIRE, England and PITTSBURGH – August 08, 2017 – Mylan Pharmaceuticals Private Limited, a subsidiary of Mylan N.V. (NASDAQ, TASE: MYL), a leading global pharmaceutical company, has received marketing authorization from the Drug Controller General of India (DCGI) for its antiretroviral (ARV) drug Avonza™ (TLE400). Avonza™ is a fixed-dose combination comprised of Efavirenz, Lamivudine and Tenofovir Disoproxil Fumarate Tablets, 400 mg/300 mg/300 mg, recommended by the World Health Organization (WHO) as an alternative first-line regimen for people being treated for HIV/AIDS.
|
Commenting on the launch, Mylan President Rajiv Malik said, “Developing Avonza™ and bringing it to patients with HIV in India is a continuation of our strong and sustained commitment to expanding access to affordable, high quality ARVs. Avonza™ will be available to patients at a cost that is lower than that of other current first-line ARVs. What’s more, Mylan is the first to offer this combination in India, making it another example of the innovative spirit that runs throughout our company to adapt our medicines, accelerate access and improve treatment outcomes.” |
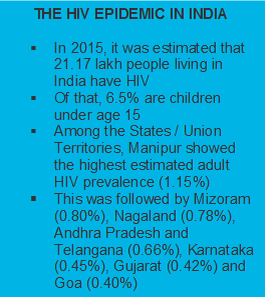 |
In April 2017, the Health Ministry in India launched the Test and Treat Policy for HIV; anyone testing positive for HIV will get antiretroviral therapy irrespective of CD4 count or clinical stage.
Mylan’s Avonza™ also is another step the company is taking to help India meet its Sustainable Development Goal of ending AIDS by 2030.
Globally, Mylan supplies life-saving ARVs to nearly 50% of patients being treated for HIV/AIDS in more than 100 developing countries. The company’s comprehensive ARV portfolio includes 14 active pharmaceutical ingredients and 50 finished dosage forms in first-line, second-line and pediatric formulations.
Mylan is a global pharmaceutical company committed to setting new standards in healthcare. Working together around the world to provide 7 billion people access to high quality medicine, we innovate to satisfy unmet needs; make reliability and service excellence a habit; do what’s right, not what’s easy; and impact the future through passionate global leadership. We offer a growing portfolio of more than 7,500 marketed products around the world, including antiretroviral therapies on which approximately 50% of people being treated for HIV/AIDS in the developing world depend. We market our products in more than 165 countries and territories. We are one of the world’s largest producers of active pharmaceutical ingredients. Every member of our more than 35,000-strong workforce is dedicated to creating better health for a better world, one person at a time. Learn more at Mylan.com.
This press release includes statements that constitute "forward-looking statements," including with regard to Avonza™ being available to patients at a cost that is lower than that of other current first-line ARVs. These statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Because such statements inherently involve risks and uncertainties, actual future results may differ materially from those expressed or implied by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to: success of clinical trials and our or our partners' ability to execute on new product opportunities; any regulatory, legal or other impediments to our or our partners' ability to bring products to market; other risks inherent in product development; the scope, timing, and outcome of any ongoing legal proceedings, including government investigations, and the impact of any such proceedings on our or our partners' businesses; actions and decisions of healthcare and pharmaceutical regulators, and changes in healthcare and pharmaceutical laws and regulations, in the United States, India and abroad; the impact of competition; strategies by competitors or other third parties to delay or prevent product introductions; the effect of any changes in our or our partners' customer and supplier relationships and customer purchasing patterns; any other changes in third-party relationships; changes in the economic and financial conditions of the businesses of Mylan or its partners; uncertainties and matters beyond the control of management; and the other risks detailed in Mylan's filings with the Securities and Exchange Commission. Mylan undertakes no obligation to update these statements for revisions or changes after the date of this release.