Update on Meridian’s Voluntary Worldwide Recall of EpiPen® Auto-Injector
If you think you may be impacted by this recall, please follow these steps:
STEP 1: Check the lot number on your carton or device to see if your EpiPen® Auto-Injector is affected by the recall.
STEP 2: If your EpiPen® Auto-Injector has been recalled, contact Stericycle at 877-650-3494 to obtain a voucher code for your free replacement product. Stericycle also will provide you with a pre-paid return package to ship the product back to Stericycle.
STEP 3: Visit your pharmacy with your voucher information to redeem your free replacement.
STEP 4: Send your recalled product to Stericycle. Do not return any devices affected by the recall until you have your replacement in hand.
Stericycle’s hours of operation are Monday-Friday 8 a.m. - 6 p.m. ET.
(last updated July 20, 3:30 PM ET)
STEP 1: Check the lot number on your carton or device to see if your EpiPen® Auto-Injector is affected by the recall.
STEP 2: If your EpiPen® Auto-Injector has been recalled, contact Stericycle at 877-650-3494 to obtain a voucher code for your free replacement product. Stericycle also will provide you with a pre-paid return package to ship the product back to Stericycle.
STEP 3: Visit your pharmacy with your voucher information to redeem your free replacement.
STEP 4: Send your recalled product to Stericycle. Do not return any devices affected by the recall until you have your replacement in hand.
Stericycle’s hours of operation are Monday-Friday 8 a.m. - 6 p.m. ET.
(last updated July 20, 3:30 PM ET)
Recalled Lots in U.S.
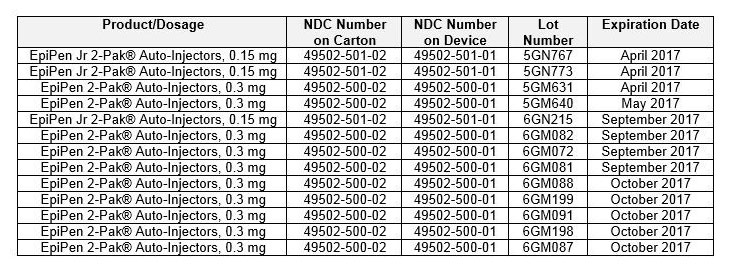
IMPORTANT NOTE: The NDC on the box ends with “2” because it contains two EpiPen Auto-injectors. The NDC on the individual EpiPen within the box has an NDC ending in “1.”

IMPORTANT NOTE: The NDC on the box ends with “2” because it contains two EpiPen Auto-injectors. The NDC on the individual EpiPen within the box has an NDC ending in “1.”
-
Confirmation of Recalled Product
 You can confirm if you are in possession of a recalled EpiPen product by checking if the lot number matches any of the lot numbers listed in the above table.
You can confirm if you are in possession of a recalled EpiPen product by checking if the lot number matches any of the lot numbers listed in the above table.
On the auto-injector, the lot number is located at the top of the label in black and preceded by the word ‘LOT.’
On the carton, the lot number is located on the left flap, which is black in color. You will find the lot number written in white and preceded by the word ‘LOT.’
If your EpiPen® Auto-Injector has been recalled, contact Stericycle at 877-650-3494 to obtain a voucher code for your free replacement product. If not, there is no further action necessary.

-
Product Replacement Voucher Program
Stericycle will provide you with a voucher code to redeem a free replacement product. If you called before Monday, April 3, Stericycle will follow-up with your voucher code via phone or email beginning Tuesday, April 4.
Each voucher is valid for one EpiPen 2-Pak or EpiPen Jr 2-Pak, or one of Mylan’s authorized generic for EpiPen 2-Pak or EpiPen Jr 2-Pak. Stericycle will issue one voucher for every two-pack that needs to be replaced. Each two-pack carton contains two auto-injectors.
After you have received your voucher, follow these steps:
STEP 1: Call your pharmacy to inform them that you have a voucher for your free replacement of the product.
STEP 2: Confirm with your pharmacy that you have a fillable prescription for EpiPen or Mylan’s authorized generic for EpiPen on file.
STEP 3: Confirm that the pharmacy has the medication in stock.
Patients may receive either EpiPen Auto-Injector or Mylan’s authorized generic for EpiPen Auto-Injector at the pharmacy as a replacement based on availability. The authorized generic has the exact same drug formulation, has the exact same operating instructions and is therapeutically equivalent to EpiPen Auto-Injector, and may be substituted for EpiPen Auto-Injector.
Mylan is committed to replacing recalled devices at no cost and Mylan would like to reassure patients that there will be no additional replacement-related financial burden to them as a result of this recall.
-
Product Return Process
If you are in possession of a recalled product, call Stericycle at 877-650-3494 to provide you with a pre-paid return package to ship the product back to Stericycle.
IMPORTANT: Do not return any devices affected by the recall until you have redeemed your free replacement from your pharmacy and have your replacement in hand.
-
Return Process for EpiPen4Schools® Participants
The EpiPen4Schools program proactively tracks the lot numbers of the product distributed to all schools enrolled in the program and has identified the schools that have EpiPen and/or EpiPen Jr Auto-Injectors affected by the recall.
Schools that we have identified as having received product subject of the recall will be notified by email and first-class mail. Replacement product will be automatically sent to those schools. Schools do not need to take any action to initiate this process.
Schools that we have identified as NOT having received product subject of the recall will receive an email indicating such with additional details about the recall. Out of an abundance of caution, those schools will be directed to check the lot numbers of the EpiPen Auto-Injectors provided by the EpiPen4Schools program. If the devices are included in the recalled lots, schools should contact BioRidge immediately at 973-845-7600.
BioRidge is Mylan’s third-party administrator of the EpiPen4Schools program.
-
Expanded Voluntary Worldwide Recall of EpiPen® Auto-Injector
Mylan announced on March 31, 2017, that Meridian Medical Technologies, a Pfizer company and Mylan’s manufacturing partner for EpiPen® Auto-Injector, has expanded a voluntary recall of select lots of EpiPen (epinephrine injection, USP) and EpiPen Jr® (epinephrine injection, USP) Auto-Injectors to now include additional lots distributed in the U.S. and other markets in consultation with the U.S. Food and Drug Administration (FDA).
This recall is being conducted as a result of the receipt of two previously disclosed reports outside of the U.S. of failure to activate the device. Both reports are related to the single lot that was previously recalled by Meridian. The incidence of the defect is extremely rare and testing and analysis across the potentially impacted lots has not identified any units with a defect. However, the recall is being expanded by Meridian to include additional lots as a precautionary measure out of an abundance of caution.
The recall impacts certain lots of the 0.3 mg and 0.15 mg strengths of EpiPen Auto-Injector. None of the recalled lots include the authorized generic for EpiPen Auto-Injector, which is also manufactured by Meridian Medical Technologies.
The recalled product was manufactured and distributed by Meridian between December 2015 and July 2016. The expanded voluntary recall is being initiated in the U.S. The recall also will extend to additional markets in Europe, Asia, North and South America.
Epinephrine is the first-line treatment for a life-threatening allergic reaction (anaphylaxis) and access to this product is critical in the event of an emergency. Delays in epinephrine administration have been associated with negative health consequences.
More information about the risks and benefits of EpiPen® Auto-Injector can be found at EpiPen.com.
-
Recalled Lots in the U.S.

NOTE: The NDC on the box ends with “2” because it contains two EpiPen Auto-injectors. The NDC on the individual EpiPen within the box has an NDC ending in “1.”
-
Press Release – English and Spanish
-
Product Images


Stericycle
To return your product, please contact Stericycle at 877-650-3494.
Mylan Customer Relations
800.796.9526
customer.service@mylan.com
Note: Contact Stericycle for the return and replacement of recalled product.
Media Relations
724.514.1968
Communications@mylan.com
Investor Relations
724.514.1813
InvestorRelations@mylan.com
Please come back to this site for any updates and additional information on the product return and replacement process.
To return your product, please contact Stericycle at 877-650-3494.
Stericycle’s hours of operation are Monday-Friday 8 a.m. - 6 p.m. ET.
Mylan Customer Relations
800.796.9526
customer.service@mylan.com
Note: Contact Stericycle for the return and replacement of recalled product.
Media Relations
724.514.1968
Communications@mylan.com
Investor Relations
724.514.1813
InvestorRelations@mylan.com
Please come back to this site for any updates and additional information on the product return and replacement process.